Ortho Meta Para Nmr Coupling
Ortho-H H H H meta-H H para-Table 4 NMR data for C11H15NO2 number of. 1-X2-Y-C 6 H 4.
J para 0-1 Hz.

Ortho meta para nmr coupling. 2 TECH 711Using Nuclear Magnetic Resonance Spectroscopy to Identify an Unknown Compound. The expansion shows the spin-spin coupling pattern arising from the para-fluorine coupling to the 2 meta-fluorine and 2 ortho proton nuclei. This is referred to as meta or 4J coupling.
Although there is not an example in this molecule if two protons are three carbons apart they are para. For para-dichlorobenzene there should be only one signal. 4 A small signal will be observed for the ipso-carbon C 1 the carbon with the ligand directly attached a medium sized signal for the para C-atom C 4 and two tall peaks for the ortho C-atoms C 2 and meta C-atoms C 3.
22 Coupling with a Benzene Ring with Three Substituents. 12 Meta-Coupling Has a Low J-Value. To a simple first order approximation the appearance of the signals for all 4 1H-atoms are readily predictable.
The fine text which you cant read sorry about that gives the coupling constants for the system which are bog-standard orthometapara couplings. 472008 Identifying Meta coupling in a 1H NMR Spectrum. In a substituted benzene ring aromatic protons that are in the meta position can exhibit coupling to each other.
Fluorine-19 nuclear magnetic resonance spectroscopy fluorine NMR or 19 F NMR is an analytical technique used to detect and identify fluorine-containing compounds. The para-coupling 5 J for both signals should be between 00 and 10 Hz. 3232021 The chemical shift difference between ortho meta and para hydrogens in such compounds is often so small that they are seen as a single resonance signal in an NMR spectrum.
23 Coupling of Protons with Meta-Substituents. Jortho 7-10 Hz Jmeta 1-3 Hz J para 0-1 Hz Example. 3132008 If you consider ortho-dichlorobenzene you would observe two signals in the 1H-NMR because there are two magnetically distinct types of protons in this molecule.
Depending on the exact value of the three ortho couplings and the two meta couplings the signals could have slightly different appearances. Ortho hydrogens on a benzene ring couple at 6-10 Hz while 4-bond coupling of up to 4 Hz is sometimes seen between meta hydrogens. 1 Different Peak Coupling in Ortho and Meta Positions.
Case b assuming chemical shift differences much larger than coupling constants. Trum arises from a phenomenon called spinspin coupling in which a given proton is affected by the spin states of protons on adjacent carbon. On a high field instrument one finds this Jortho as well as a Jmeta and a J from the effect of the protons meta and para to it Typically.
The ortho-coupling 3 J for both signals should be about 60 and 90 Hz. The 2-bond coupling between hydrogens bound to the same alkene carbon referred to as geminal hydrogens is very fine generally 5 Hz or lower. 13C NMR study of ortho meta and para substituted phenyldiphenylamines.
These numbers are from the Hesse-Meier-Zeeh a German textbook about spectroscopy in organic chemistry. Substituent effect correlations April 2005 Magnetic Resonance in Chemistry 153296 - 302. For instance if J ab.
The only variable in the simulations is the chemical shift of the downfield peak which slowly moves towards its upfield partner. Ortho typically is 7 10 Hz while J meta is a smaller 2 3 Hz for these. The meta-coupling 4 J for both signals should be between 09 and 30 Hz.
1152010 It is more difficult to identify between ortho and meta isomers without more knowledge of aromatic splitting patterns and couplings. The general couplings can be explained with the help of the benzene diagram below with the strength of coupling decreasing from Ortho. 21 Benzene Ring with Only Ortho Coupling.
H a and H b are ortho to one another adjacent. 1011973 The root-mean-square deviations between the observed and calculated line positions of the ortho meta and para compound were 055 092 and 091 Hz respectively. J ortho 6-10 Hz.
24 Coupling with Ortho-Substituents. Hortho Hmeta Hpara H Jortho Jmeta J para In low-field 1H NMR the signal for this proton would be split into a doublet by the proton ortho to it. 6-9 Hz NMR AROMATIC PROTON COUPLING In aliphatic organic compounds the only coupling that you need to worry about is from adjacent protons 0 between any non-adjacent protons.
The 90 MHz spectrum of benzyl alcohol in chloroform-d solution provides an. Most aromatic compounds such as drugs have a substituted benzene ring with electron withdrawing and donating groups therefore all these factors lead to a very complex NMR but also important for. For a mono-substituted ring four signals are observed in the 13C-NMR spectrum because there is a symmetry plane passing through C 1 and C.
In aromatic compounds however significant splitting does not only come from ortho protons coupled to each other but also from meta even para protons. In some instances the meta isomer can be identified by. J meta 1-3 Hz.
H a and H c are meta to one another two carbons apart. 11 Ortho-Couplings Have a High J-Value. The values of the direct couplings and the chemical shifts obtained in this manner are given in table 1 together with the values used for the indirect couplings.
The coupling pattern is typically a doublet with a coupling. 2 Check Benzene Ring coupling for Each Case. For meta- there should be three signals.

19 F A 1 H Pure Shift Bird B And 1 H C Spectra Of Download Scientific Diagram

Nmr Spectroscopy Lecture 5 Substitution Patterns On Aromatic Rings Part 5 Of 5 Youtube
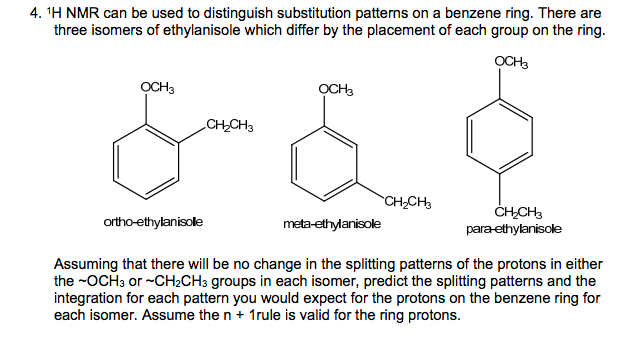
Solved 4 1h Nmr Can Be Used To Distinguish Substitution Chegg Com

How Can Multiplets In Para Disubstituted Benzene Rings Be Described Chemistry Stack Exchange

1h Nmr Of P Methoxy Phenol 32 Possible Lines Chemistry Stack Exchange
Komentar
Posting Komentar