Ortho Meta Para Proton Nmr
Groups considering benzene as a aromatic substrate. DE LANGE Scheikundig Laboratorium der Vrije Universiteit Amsterdam The Netherlands Received 28 June 1973 Proton NMR spectra of the dicyano benzenes dissolved in.

Ch 2 Ch 2 Regions Of The 600 Mhz 1 H Nmr Spectra Of The Isomeric Download Scientific Diagram
Ortho coupling is the strongest at 15 Hz Meta follows with an average of 2 Hz and finally para coupling is usually insignificant for studies.
Ortho meta para proton nmr. The CDCl 3 peak is pointed out in each spectrum. Ortho parallel proton spins and para antiparallel spins. The types of NMR usually done with nucleic acids are 1 H or proton NMR 13 C.
As you can see the J value decreases as the number of bonds between hydrogens increases. DE KIEVIET and CA. Ratios of interproton distances have been determined in each case.
Ortho-Couplings Have a High J-Value. Indeed hydrogen gas H 2 exists as two stable spin isomers. For instance if J ab.
Nuclear magnetic resonance spectroscopy. NMR Spectroscopy 1 Chemical shifts. You can use 13C NMR for distinguishing o-m- and p-sub.
1152010 The para isomer is the most distinct as this gives a symmetrical compound. The samples were run using CDCl 3 as the solvent and a small contaminant of this deuterated solvent is CHCl 3 which shows up at 724 ppm. 3132008 If you consider ortho-dichlorobenzene you would observe two signals in the 1H-NMR because there are two magnetically distinct types of protons in this molecule.
Spectrum and that should be a much clearer indicator than your IR spectra as to whether you made the ortho or the para isomer. Although there is not an example in this molecule if two protons are three carbons apart they are para. The shifts of protons ortho meta or para to a substituent.
13 C chemical shift correlations for carbons meta and para to the substituent were not significantly better than when Q was omitted. Examples of ortho meta and para substitution are illustrated in the NMR spectra of different isomers of chloronitrobenzene below. On an aromatic.
1242016 You should also consider your mathbf13 C NMR spectra as that would tell you more. 6-9 Hz NMR AROMATIC PROTON COUPLING In aliphatic organic compounds the only coupling that you need to worry about is from adjacent protons 0 between any non-adjacent protons. For para-dichlorobenzene there should be only one signal.
The more symmetry you have on the molecule the less number of carbons would appear to show up on the 13 C. 1011973 Volume 22 number 2 CHEMICAL PHYSICS LETTERS 1 October 1973 THE NMR SPECTRA OF ORTHO META AND PARA DICYANO BENZENE IN A NEMATIC SOLVENT W. The closer the proton is to the other hydrogen atoms the greater the effect on the proton.
Substituent Ortho Meta Para NO2 095 017 033 CHO 058 021 027 COCI 083 016 03 COOH 08 014 02 COOCH3 074 007 02. Although a 2D COSY experiment can produce the same result one can save time by looking for this information in a 1H NMR spectrum first. Most aromatic compounds such as drugs have a substituted benzene ring with electron withdrawing and donating groups therefore all these factors lead to a very complex NMR but also important for drug discovery and analysis.
The hydrogen at the para-position of the benzene ring is unaffected by coupling. Are ortho to one another adjacent. Nmr spectroscopy is normally carried out in a liquid phase solution or neat so that there is close contact of sample molecules with a rapidly shifting crowd of other molecules Brownian motion.
H a and H c are meta to one another two carbons apart. Illustrated below is a portion of a 1H NMR spectrum for a substituted benzene ring. Significant correlations were obtained between field and resonance parameters and 13 C chemical shifts of C o and C p and C i C o C m and C p of the nonsubstituent bearing phenyl rings in ortho and para substituted.
J para 0-1 Hz. J meta 1-3 Hz. J ortho 6-10 Hz.
Popular Answers 1 25th Mar 2014. J-coupling can be used to identify ortho-meta-para substitution of a ring. Ortho typically is 7 10 Hz while J meta is a smaller 2 3 Hz for these.
To a simple first order approximation the appearance of the signals for all 4 1H-atoms are readily predictable. Accurate values for the indirect couplings and chemical shifts of ortho and meta dicyano benzene have been obtained in acetone. 472008 On the contrary a spectrum without any meta coupling indicates a lack of protons in the meta position.
In which the protons in the ortho and para positions are more strongly shielded than in the meta position. The fine text which you cant read sorry about that gives the coupling constants for the system which are bog-standard orthometapara couplings. For ortho groups of an aromatic molecule will give 3 signals for Carbons.
Depending on the exact value of the three ortho couplings and the two meta couplings the signals could have slightly different appearances. The general couplings can be explained with the help of the benzene diagram below with the strength of coupling decreasing from Ortho. In aromatic compounds however significant splitting does not only come from ortho protons coupled to each other but also from meta even para protons.
You would expect to see 2 peaks each integrating to 2H and each appearing as a. 1011973 Proton NMR spectra of the dicyano benzenes dissolved in a nematic liquid crystal have been measured and analysed. For meta- there should be three signals.
So we can ignore it but we need to understand how the proton hydrogen shows up in the ortho and meta positions. The only variable in the simulations is the chemical shift of the downfield peak which slowly moves towards its upfield partner.

Para Dichlorobenzene Number Of Proton Nmr Signals Chemistry Stack Exchange

Aromatic Region Of 1 H Nmr Spectra For Deuterated Benzene Solutions Of Download Scientific Diagram

1h Nmr 11 2 D 2015 11 2 E 2015 Notes Kychem
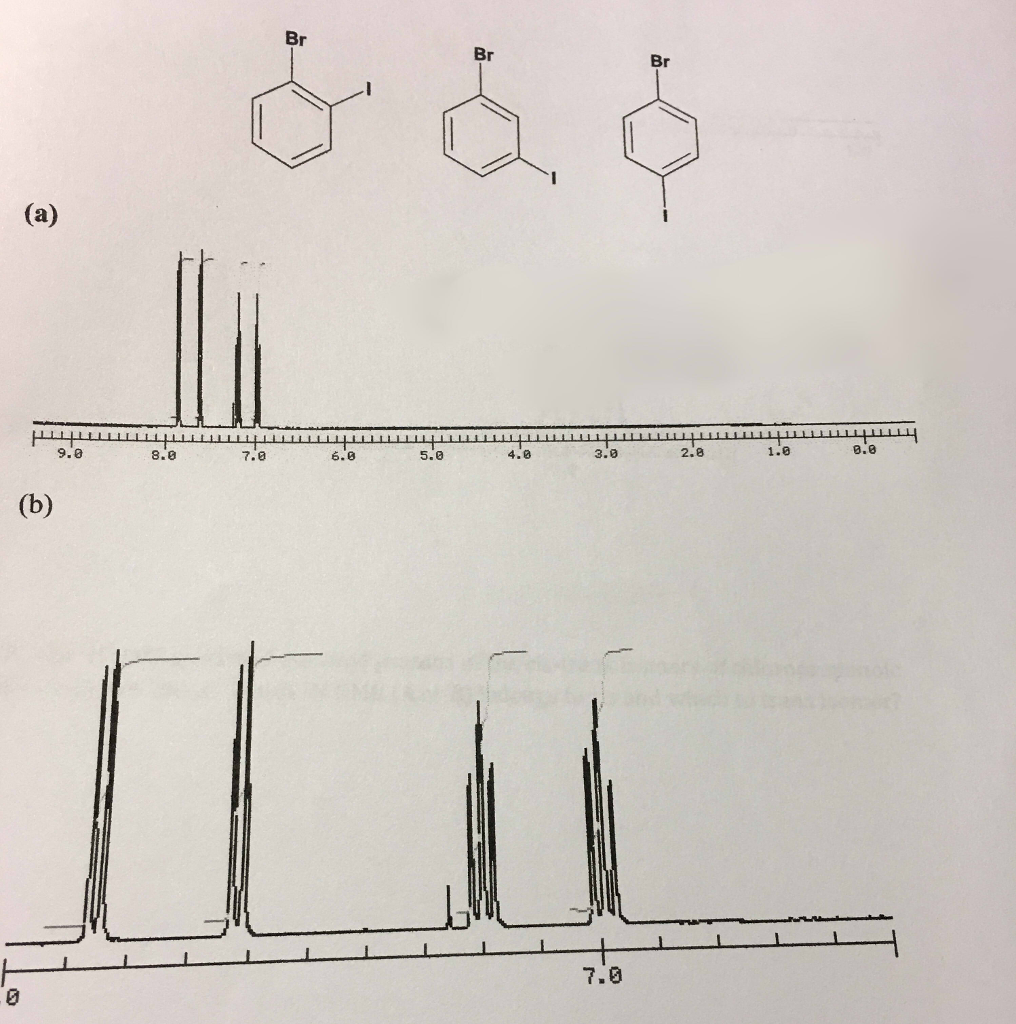
Solved From This Nmr How Do You Tell If The Compound Is O Chegg Com
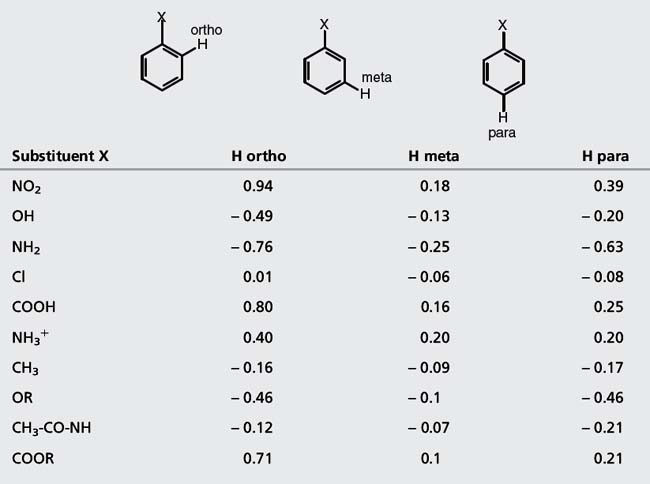
Nuclear Magnetic Resonance Spectroscopy Basicmedical Key
Komentar
Posting Komentar