The Strongest Ortho Para And Meta Directing Groups
1292018 Examples of ortho- para directors are hydroxyl groups ethers amines alkyl groups thiols and halogens. The reaction tolerates a wide array of orthoparadirecting groups such as F Cl Br CH 3 Et iPr OCH 3 and OCF 3.
Why Are Halogens Ortho Para Directors Master Organic Chemistry
Option A is correct.
The strongest ortho para and meta directing groups. Bonded to the ring are powerful activating groups and are strong orthopara directors. Common meta directing groups. Asked May 21 2019 in Chemistry by ManishaBharti 650k points.
Our results do not support that orthopara directing groups are electron donors and meta directing groups are electron acceptors. Ortho para directing groups are electron-donating groups. The nitration of methoxybenzene also known as anisole.
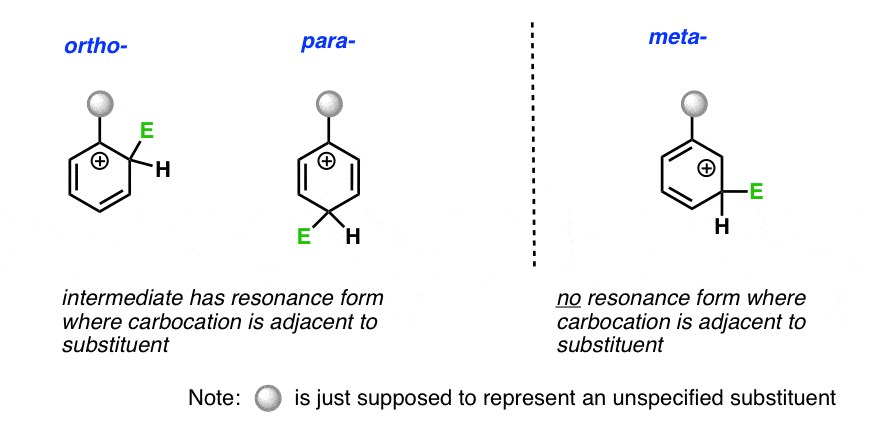
Therefore -NH 2 group is an ortho-para director. See also activating group deactivating group. In both ortho and para substitutions there is a resonance structure with a tertiary carbocation stabilized by stronger hyperconjugation.
Heres a concrete example. Your question has some confusion in the concepts. Groups with unshared pairs of electrons such as the amino group of aniline are strongly activating and orthopara-directing by resonance.
Ortho- and para- products dominate while meta products comprise less than 3. Common ortho para directing groups. It is a meta-directing group and a deactivating group because the rm -I effect of the group is quite strong and significantly outweighs that stabilization caused by conjugation that you can describe with resonant structures.
SOME ORTHO PARA DIRECTORS. Groups with an oxygen or nitrogen attached to the aromatic ring are ortho and para directors since the O or N can push electrons into the ring making the ortho and para positions more reactive and stabilizing the arenium ion that forms. Ortho meta and para.
A ortho-para-directing group is not necessary an activating group. Common meta directing groups. The group which directs the second incoming group to the meta position is called a meta-director.
Deactivators not halogens are meta-directing. This causes the ortho and para products to form faster than meta. Halogens -NH 2 -R -OH.
Electron donating groups are generally orthopara directing for electrophilic aromatic substitution while electron withdrawing groups are generally meta directors with the exception of the halogens which are also orthopara directors as they have lone. All the functional groups are divided into ortho - para or meta -directors. To which one the group belongs depends on how it stabilizes or destabilizes the transition state.
Ortho para directing groups are electron-donating groups. Thus the nitro group is a meta directing group. The halide ions which are electron-withdrawing but ortho para directing are the exception.
Common ortho para directing groups. OrthoPara-Directing Groups and Meta-Directing Groups. 11182014 Orthopara directing groups have most negative charges on the orthopara positions while meta directing groups often possess the largest negative charge on the meta position.
There are three relative positions for a disubstituted benzene ring. Deactivators halogens are ortho-para directing. All activators are ortho-para directors.
OH is strongest ortho para directing group. Deactivates decreases electron density on benzene ring so electrophile can attack on meta position with difficulty. Now the order Meta directing.
8242020 Thus the methyl group is an ortho para directing group. Ortho meta and para historically carried different meanings but in 1879 the American Chemical. Meta directing groups are electron-withdrawing groups.
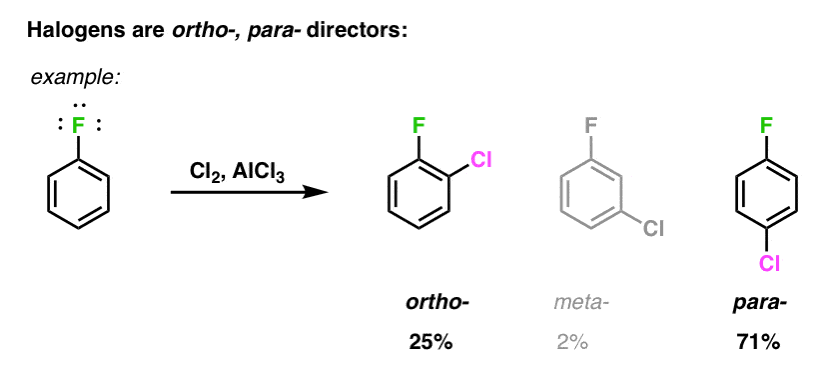
The -OH group tends to be electron-donating because the extra electrons around the oxygen atom can be shared with the aromatic benzene ring. Because the -OH group is a strongly activating group see Electrophilic aromatic directing groups. Although chlorine is an electron- withdrawing group yet it is ortho and para directing in electrophillic aromatic substitution reactions.
Halogens are electronegative so they are deactivating by induction but they have lone pairs so they are resonance donors and therefore orthopara directors. And this is true for any activating group. The prefixes derive from Greek words meaning correctstraight followingafter and similar respectively.
4112019 This protocol allows the synthesis of metanitrated arenes that are tedious to prepare or require multistep synthesis using the existing methods. It also provides regioselective access to the nitro derivatives of πelectrondeficient heterocycles. Meta directing groups are electron-withdrawing groups.
Electron withdrawers deactivators have a positive charge on the substituent or a very electronegative atom attached to it which pulls electrons out of the benzene. Such activating groups donate those unshared electrons to the pi system creating a negative charge on the ortho and para. Halogen are dectivator due -I effect so their reactivity is less than of benzene but Ortho-Para directing due to R mesomeric effect.
Thus it contributes resonance structures that can be seen below. Most neutral species we studied here are electron withdrawal in. 1022019 The terms ortho meta and para are prefixes used in organic chemistry to indicate the position of non-hydrogen substituents on a hydrocarbon ring benzene derivative.
Some common ortho para directing groups are Cl -Br -I -OH -NH 2 -CH 3 -C 2 H 5. In general substituent groups with unshared electron pairs on the atom adjacent to the benzene ring eg hydroxyl amino are stronger activating groups than groups. This resonance contributor stabilizes the transition state thus making the ortho and para substitution faster than meta substitution.
The halide ions which are electron-withdrawing but ortho para directing are the exception.

Ortho Para Meta In Eas With Practice Problems Chemistry Steps

Why Are Halogens Ortho Para Directors Master Organic Chemistry

The Strongest Ortho Para And The Strongest Meta Directing Groups

Ortho Para Meta In Eas With Practice Problems Chemistry Steps

The Strongest Ortho Para And The Strongest Meta Directing Groups
Komentar
Posting Komentar