Para Position Of Monosubstituted Benzene
Instead of using numbers to indicate substituents on a benzene ring ortho- o- meta- m- or para p- can be used in place of positional markers when there are two substituents on the benzene ring disubstituted benzenes. 02102019 Updated October 02 2019.

Orientation Of Products Substituent In Monosubstituted Benzene Derivatives Ortho Meta Para Substitution Resonance Structures Hybrid Methylbenzene Chlorobenzene Phenol Nitrobenzene Benzoic Acid Effect On Synthesis Routes Advanced A Level Organic
16052020 Some examples of directive influence of functional group in mono substituted benzene are explained below.
Para position of monosubstituted benzene. For example chlorine Cl attached to a phenyl group would be named chlorobenzene chloro benzene. Due to the resonance in the benzene ring electron density at ortho and para position increases as compared to the meta position. If substitution were totally random an orthometapara product ratio of 221 would be expected.
These groups direct the electrophilic attack on. All activating groups are ortho-para directors for example NH 2 NHR NHCOCH 3 OCH 3 CH 3 C 2 H 5 etc. The NMR of bromobenzene is shown below.
Trimethylsilyl tert-butyl and isopropyl groups can form stable carbocations hence are ipso directing groups. It is observed in compounds such as calixarenes and. Key Notes ortho meta and para substitution.
Since there is only one substituent on the benzene ring we do not have to indicate its position on the benzene ring as it can freely rotate around and you would end up getting the same compound. Ortho 2 positions. Ipso-substitution describes two substituents sharing the same ring position in an intermediate compound in an electrophilic aromatic substitution.
These positions are defined as the ortho meta and para positions. The terms ortho meta and para are prefixes used in organic chemistry to indicate the position of non-hydrogen substituents on a hydrocarbon ring benzene derivative. They may be present on the benzene rings which direct attack according to their preference such as ortho para position.
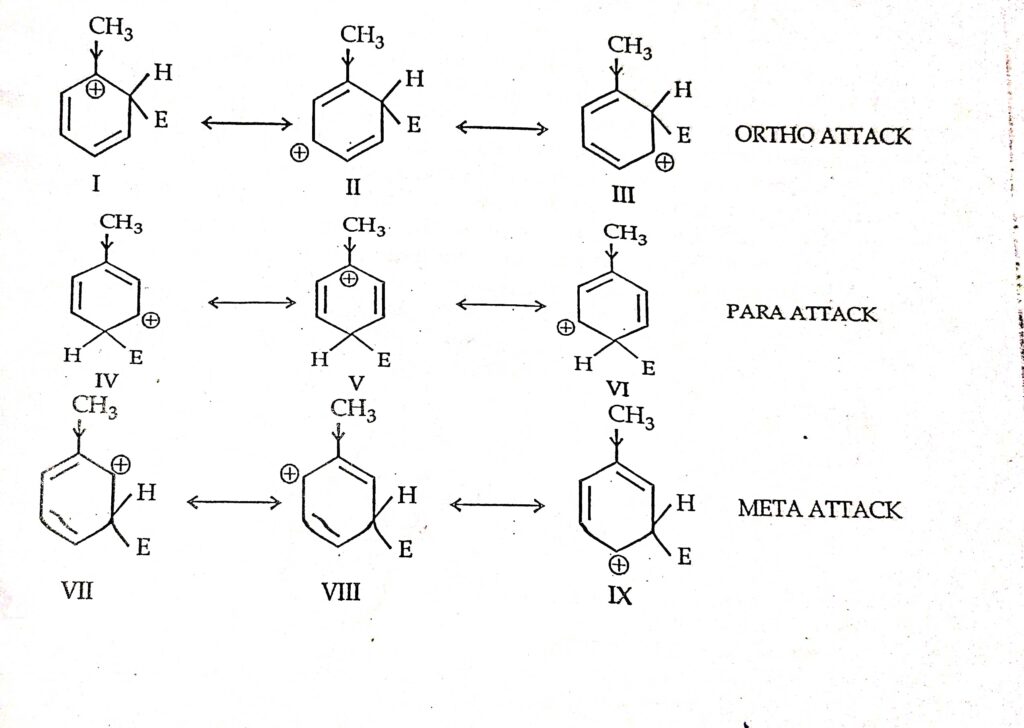
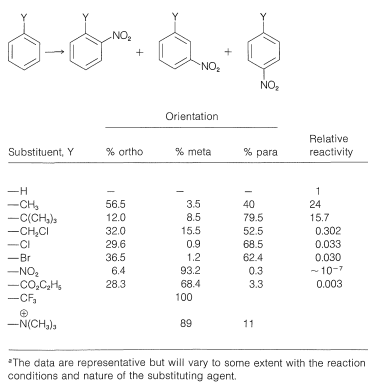
The sigma complex or carbocation intermediate is a resonance hybrid of three Structure. Classify each of the substituents listed in Objective 2 of Section 164 as being either meta or orthopara directing. 35 The group already on the ring determines which position the new group will take and whether the reaction will be slower or faster than with.
Notice the peaks that are shifted downfield 74-75 ppm. Meso-substitution refers to the substituents occupying a benzylic position. Ortho- Meta- Para- OMP Nomenclature for Disubstituted Benzenes.
The second substituent may enter the mono-substituted benzene ring at either ortho para or at meta position. ELECTROPHILIC SUBSTITUTIONS OF MONO-SUBSTITUTED AROMATIC RINGS. From the figure.
If reaction occurs equally well at all available sites the expected statistical mixture of isomeric products would be 40 ortho 40 meta and 20 para. Meta 2 positions. 01032021 There are different type of substituents.
If the monosubstituted benzene contains an electron-withdrawing group such as Br or Cl the protons adjacent to the electron-withdrawing group will be shifted slightly downfield of the other aromatics producing separate peaks. 10092020 Lets study the sigma complexes carbocation intermediate which are formed by attacked of electrophile E at ortho para and meta position of mono substituted benzene toluene. Positions of substituents if 1- di tri substituent n benzene.
Ortho meta and para historically carried different. 11 rows When one of the positions on the ring has been substituted with another atom or. For example nitration of bromoben-zene could in principle give ortho-meta- or para-bromonitrobenzene.
Reason The position of the incoming group is determined by the nature of the group present in monosubstituted benzene ring. The prefixes derive from Greek words meaning correctstraight followingafter and similar respectively. When an electrophilic substitution reaction is performed on a monosubstituted benzene the new group may be directed primarily to the ortho meta or para position and the substitution may be slower or faster than with benzene itself.
As discussed earlier these groups direct the electrophilic attack on ortho and para positions. In monosubstituted benzene C 6 H 5 -G there are five hydrogen atoms. Since a mono-substituted benzene ring has two equivalent ortho-sites two equivalent meta-sites and a unique para-site three possible constitutional isomers may be formed in such a substitution.
But this distribution is never obtained. When a monosubstituted benzene undergoes an electrophilic aromatic substitution reaction three possible disubstitution products might be obtained. Para 1 position.
They are defined as the following. 23092020 draw the resonance contributors for the carbocation intermediate formed during the reaction of a given monosubstituted benzene derivative with any of the electrophiles discussed in this chapter. Electrophilic substitution can occur at three different positions on a mono-substituted aromatic ring.
On the basis of above position one way to classify substituent as ortho-para directing or. The products formed in factis determined by the nature of the first substituent already present on the ring. As a result halogens too are ortho-para directors irrespective of the fact that they are deactivators due to -I effect.
The activating group directs the reaction to the ortho or para position which means the electrophile substitute the hydrogen that is on carbon 2 or carbon 4. These H a couple to H b J.

Orientation Of Products Substituent In Monosubstituted Benzene Derivatives Ortho Meta Para Substitution Resonance Structures Hybrid Methylbenzene Chlorobenzene Phenol Nitrobenzene Benzoic Acid Effect On Synthesis Routes Advanced A Level Organic
22 5 Effect Of Substituents On Reactivity And Orientation In Electrophilic Aromatic Substitution Chemistry Libretexts

Orientation Of Products Substituent In Monosubstituted Benzene Derivatives Ortho Meta Para Substitution Resonance Structures Hybrid Methylbenzene Chlorobenzene Phenol Nitrobenzene Benzoic Acid Effect On Synthesis Routes Advanced A Level Organic

Komentar
Posting Komentar