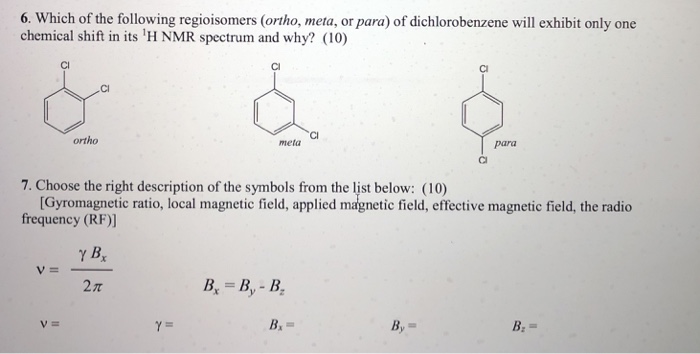
Nmr Ortho Meta Para
Although there is not an example in this molecule if two protons are three carbons apart they are para. 13 C NMR spectra of 37 ortho meta and para substituted phenyl benzoates containing substituents in benzoyl and phenyl moiety 4 ortho substituted methyl and 5 ethyl benzoates as well as 9 Rsubstituted alkyl benzoates have been recorded.
Solved 6 Which Of The Following Regioisomers Ortho Met Chegg Com
Substituent effect correlations Eugene Grimley.

Nmr ortho meta para. To a simple first order approximation the appearance of the signals for all 4 1H-atoms are readily predictable. Accurate values for the indirect couplings and chemical shifts of ortho and meta dicyano benzene have been obtained in acetone. In the previous post we saw that a benzene ring with an activator undergoes electrophilic aromatic substitution at the ortho and para positions while deactivated aromatic rings react at the meta position.
Ratios of interproton distances have been determined in each case. The fine text which you cant read sorry about that gives the coupling constants for the system which are bog-standard orthometapara couplings. J ortho 6-10 Hz.
NMR Coupling of Benzene Rings. 13 C NMR study of ortho meta and para substituted phenyldiphenylamines. Why is the trend so different for the carbon-13 NMR.
Furthermore the ortho protons are closer to the nitro group hence they are the most deshielded of all so the from more to less deshielded. You would expect to see 2 peaks each integrating to 2H and each appearing as a doublet due to coupling to adjacent proton. The only variable in the simulations is the chemical shift of the downfield peak which slowly moves towards its upfield partner.
Ortho typically is 7 10 Hz while J meta is a smaller 2 3 Hz for these. H a and H c are meta to one another two carbons apart. As you can see the J value decreases as the number of bonds between hydrogens increases.
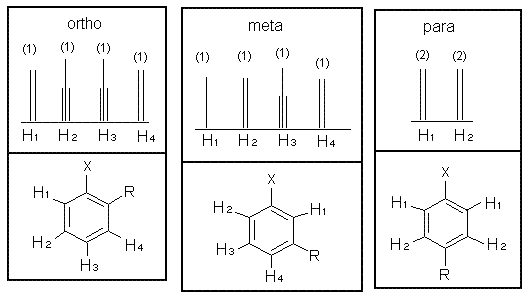
For a mono-substituted ring four signals are observed in the 13C-NMR spectrum because there is a symmetry plane passing through C 1 and C. Depending on the exact value of the three ortho couplings and the two meta couplings the signals could have slightly different appearances. For instance if J ab.
Ortho-Meta Peak and Chemical Shifts NMR Nuclear Magnetic Resonance is important when determining the structure of a compound. Examples of ortho meta and para substitution are illustrated in the NMR spectra of different isomers of chloronitrobenzene below. For meta- there should be three signals.
So from more to less deshielded the positions are now ipso. 1242016 MORE SYMMETRY REDUCES THE CHANGE IN DIPOLE MOMENT The vector direction of the nitro group stretches on the para isomer is more symmetrical with respect to the vector direction of the hydroxyl group stretches than in the ortho isomer which reduces the overall change in dipole moment during those stretches thus weakening any peaks pertaining to those. The chemical shift difference between ortho meta and para hydrogens in such compounds is often so small that they are seen as a single resonance signal in an nmr spectrum.
4 A small signal will be observed for the ipso-carbon C 1 the carbon with the ligand directly attached a medium sized signal for the para C-atom C 4 and two tall peaks for the ortho C-atoms C 2 and meta C-atoms C 3. 1152010 The para isomer is the most distinct as this gives a symmetrical compound. Ortho Para and Meta in Disubstituted Benzenes.
For para-dichlorobenzene there should be only one signal. J para 0-1 Hz. For ortho groups of an aromatic molecule will give 3 signals for Carbons.
Are ortho to one another adjacent. The para-substitution NMR aromatic region pattern usually looks quite different than the patterns for both ortho-and meta-substituted aromatic rings. J meta 1-3 Hz.
However the reported 13 C NMR spectrum shows signals at 1483 ipso 1347 para 1294 meta 1235 ortho. The 90 MHz spectrum of benzyl alcohol in chloroform-d solution provides an instructive example shown below. This Organic Chemistry video is about HNMR of Bezene Why Ortho meta and Para carbons differ.
Department of Chemistry Mississippi State University Mississippi State Mississippi 39762 USA. DE LANGE Scheikundig Laboratorium der Vrije Universiteit Amsterdam The Netherlands Received 28 June 1973 Proton NMR spectra of the dicyano benzenes dissolved in. 1011973 Volume 22 number 2 CHEMICAL PHYSICS LETTERS 1 October 1973 THE NMR SPECTRA OF ORTHO META AND PARA DICYANO BENZENE IN A NEMATIC SOLVENT W.
DE KIEVIET and CA. How ortho para meta groups of. 3132008 If you consider ortho-dichlorobenzene you would observe two signals in the 1H-NMR because there are two magnetically distinct types of protons in this molecule.
You can use 13C NMR for distinguishing o-m- and p-sub. Groups considering benzene as a aromatic substrate. By studying the environment of the protons hydrogen you will be able to guess the structural formula.
1011973 Proton NMR spectra of the dicyano benzenes dissolved in a nematic liquid crystal have been measured and analysed. And the protons on the ortho and para positions will be shielded by electrons and showed up at less ppm value in the 1H NMR. It is more difficult to identify between ortho and meta isomers without more knowledge of aromatic splitting patterns and couplings.

Nmr Spectroscopy Lecture 5 Substitution Patterns On Aromatic Rings Part 5 Of 5 Youtube

1h Nmr Of P Methoxy Phenol 32 Possible Lines Chemistry Stack Exchange

Protons On Aromatic Rings In Nmr Chemistry Stack Exchange

Ch 2 Ch 2 Regions Of The 600 Mhz 1 H Nmr Spectra Of The Isomeric Download Scientific Diagram
Komentar
Posting Komentar