Ortho Meta And Para Ratio In Monosubstituted Benzene Ring Is
When an electrophilic substitution reaction is performed on a monosubstituted benzene the new group may be directed primarily to the ortho meta or para position and the substitution may be slower or faster than with benzene itself. Are ortho to one another adjacent.
Learn Disubstitution Of Aromatic Compounds Meaning Concepts Formulas Through Study Material Notes Embibe Com
The prefixes derive from Greek words meaning correctstraight followingafter and similar respectively.

Ortho meta and para ratio in monosubstituted benzene ring is. 35 The group already on the ring determines which position the new group will take and whether the reaction will be slower or faster than with benzene. Of the three possible isomers arising from the bromination of toluene only two the ortho and para are formed in significant quantity. Although there is not an example in this molecule if two protons are three carbons apart they are para.
In the case of the ortho-monosubstituted POABCS the kinetic mobility of the azobenzene mesogen is very limited due the small bond angle which disturbs the order packaging of monosubstituted. Ortho meta and para historically carried different meanings but in 1879 the American Chemical. At the same time a substituent can also be a meta director or an orthopara director.
J meta 1-3 Hz. Both methods can be used when the parent is a. As a reminder the ortho- meta and para are the relative positions of the two groups in a disubstituted aromatic ring.
If the opposite is observed the substituent is called a. 7142 The effect of an activating group on substitution orientation in a monosubstituted benzene derivative - 2 4 and 6 position directing groups ortho and para substitution positions. 1 using the descriptors ortho meta and para or 2 using locants ie 13 is the same as meta.
The products formed in factis determined by the nature of the first substituent already present on the ring. If the opposite is observed the substituent is called a meta directing group. 14- across from each other in a benzene ring.
14- across from each other in a benzene ring. Click to see full answer. The second substituent may enter the mono-substituted benzene ring at either ortho para or at meta position.
1022019 The terms ortho meta and para are prefixes used in organic chemistry to indicate the position of non-hydrogen substituents on a hydrocarbon ring benzene derivative. The mechanisms leading to these three isomers are shown in Fig. Ortho Para and Meta in Disubstituted Benzenes.
J para 0-1 Hz. If substitution were totally random an orthometapara product ratio of 221 would be expected. 12- next to each other in a benzene ring meta- m.
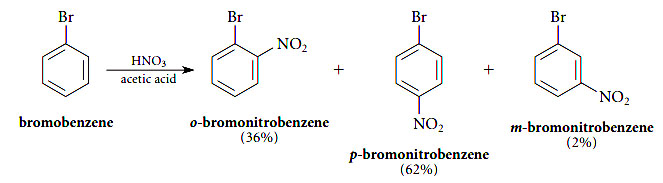
12- next to each other in a benzene ring meta- m. Other electrophilic substitution reactions of bromobenzene also give mostly ortho and para isomers. Zene could in principle give ortho-meta- or para-bromonitrobenzene.
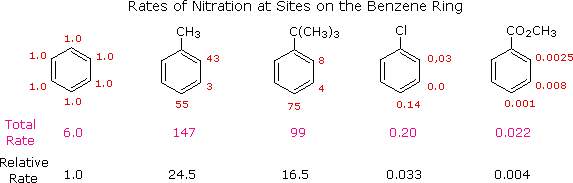
Depending on the group X that is initially present on the benzene ring the second substituent goes either to orthopara or the meta position. 672013 In comparison the bond angle between the methoxy-substituent on the azobenzene moiety and the first benzene ring of the central rigid portion of side-chain increased in the order ortho. 9232020 As you saw in Section 164 a substituent on a benzene ring can be an activator or a deactivator.
4282020 If the relative yield of the ortho product and that of the para product are higher than that of the meta product the substituent on the benzene ring in the monosubstituted benzene is called an ortho para directing group. Certain substituent groups in a benzene ring can increase the electron density of the benzene ring and make the aromatic compound more reactive towards electrophiles. 13- separated by one carbon in a benzene ring para- p.
In Electrophilic aromatic substitution in mono-substituted Benzene group already present on benzene ring decides the position of upcoming electrophile directive influence and also rate of the reaction activative -deactivative influence. In monosubstituted benzene C 6 H 5-G there are five hydrogen atoms. These isomers differ from each other in the relative positions of the methyl groups and can be named in two ways.
Dimethyl derivatives of benzene are called xylene and there are three constitutionally isomeric xylenes. Ortho 2 positions Meta 2 positions Para 1 position But this distribution is never obtained. Reason The position of the incoming group is determined by the nature of the group present in monosubstituted benzene ring.
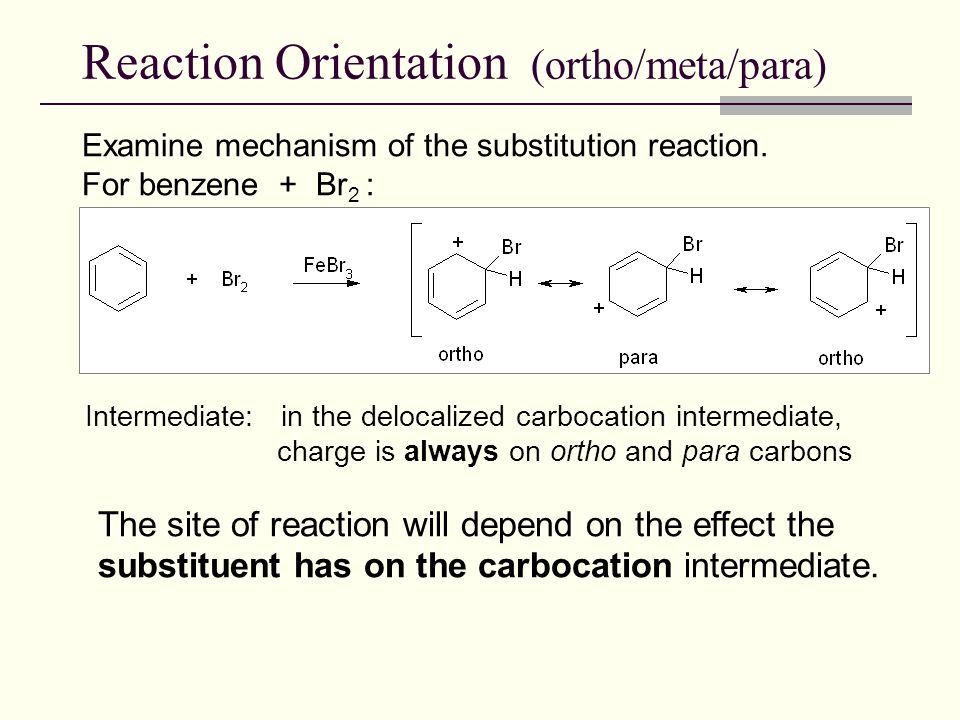
In the previous post we saw that a benzene ring with an activator undergoes electrophilic aromatic substitution at the ortho and para positions while deactivated aromatic rings react at the meta position. The aromatic ring is said to be disubstituted and the three possible isomers are described as being ortho meta and para. J ortho 6-10 Hz.
13- separated by one carbon in a benzene ring para- p. If the relative yield of the ortho product and that of the para product are higher than that of the meta product the substituent on the benzene ring in the monosubstituted benzene is called an ortho para directing group. Of the four possible combinations only three are knownthere are no meta directing activators.
H a and H c are meta to one another two carbons apart. Why It is found experimentally that this substitution is not random but is regioselective. AnaromaticringWhenthearomaticringismonosubstituedmeaningitalreadyhas onesubstituentonitthenitrogroupcanbeaddedtoeitherth eorthometaorpara positionasseeninfigure1.
Furthermore the bromination of toluene. As you can see the J value decreases as the number of bonds between hydrogens increases.

Ortho Para Meta In Eas With Practice Problems Chemistry Steps

Ortho Para Meta In Eas With Practice Problems Chemistry Steps
Reaction Orientation Ortho Meta Para Ppt Video Online Download

Ortho Para Meta In Eas With Practice Problems Chemistry Steps
Komentar
Posting Komentar