Para Nitroaniline From Aniline
And third step deacylation of nitro acetanilide. 55 matches found for para-nitroaniline.

Answer The Following Write A Reaction To Bring About The Following Conversions Aniline Into P Nitroaniline Chemistry Shaalaa Com
Advanced Search Structure Search.
Para nitroaniline from aniline. In this video I will be synthesizing p-nitroaniline from acetanilide. This chemical is used mainly as a precursor for a dye but it can also be used in an i. The correct increasing order of basic strength for the following compounds is.
It is a natural substance compound comprising of a benzene ring in which an amino gathering is para to a nitro gathering. Fast Red Base 2J. Overall there was a significant correlation r2 083 between the hemotoxicity and the Hammett constant sigmap suggesting that it is the electron-withdrawing properties of the substituent that influence the methemoglobin formation.
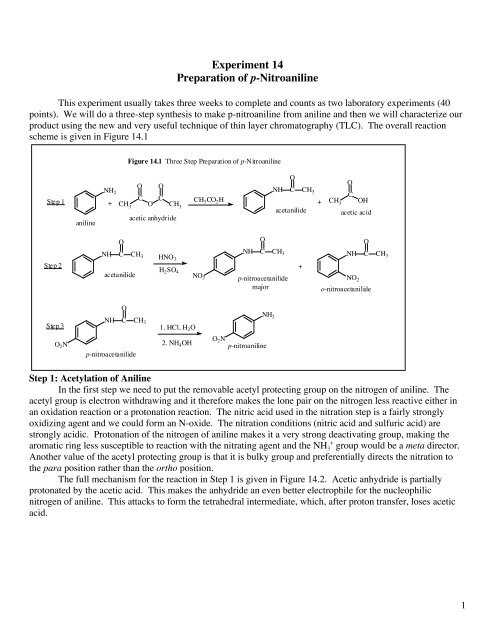
It is a derivative of aniline carrying a nitro functional group in position 2. Azoic Diazo Component 37. N H4O p- nitroa l e p- n itro ac el d acetanilide acetic anhydride acetic acid Step 1 acetanilide Step 2 Step 3 p-nitroacetanilide major o-nitroacetanilide Step 1.
Aniline when treated with acetoacetate will produce Nacetyl aniline. Aniline is a ortho para activator. PARA NITRO ANILINE Nitroaniline p-nitroaniline or 1-amino-4-nitrobenzene is a natural compound with the recipe C6H6N2O2.
2-Nitroaniline is an organic compound with the formula H 2 NC 6 H 4 NO 2. The nitronium ion will favorably do the substitution on the para position due to steric factors. A bit of ortho form will also form but it will.
Fast Red 2G Base. Preparation of p-Nitroaniline Maria Roca February 12th 2020 Aim. 3 Product Results.
The acetyl group is. M - nitroaniline. But we need only one bromine atom to connect to the para position of aniline.
The para isomer is more stable than Ortho isomer and hence the p-nitro aniline has no need to donate a pair of electron. An Unprecedented Blueshifted Naphthalimide AIEEgen for Ultrasensitive Detection of 4Nitroaniline in Water via ReceptorFree IFE Mechanism. Fast Red Base GG.
Look basicity means the ability of donating electron pairnow aniline has 1 lone pair on the N atom of NH2 groupwhich is stabilized by resonance in the benzene ring. Along with para product a trace of ortho product is also formed. Chemistry An Asian Journal 2019 14 24 4725-4731.
Study Problem 234 Outline a preparation of p-nitroaniline from aniline and any other reagents. Please select more than one item to compare. Select up to 4 products.
Therefore we reduce the activity of aniline by the reaction of. Of the anilines studied 4-nitroaniline caused the most methemoglobin 365 - 80 while aniline caused the least 03 - 05. Sothe availability of the lone pair is less.
1910B This strategy is used in the solution to Study Problem 234. Fast Red GG Base. O - nitroaniline The pKa values of m - nitroaniline p - nitroaniline and o - nitroaniline isomers are 25 10 and -03 respectively.
Naphtoelan Red GG Base. Para nitroacetanilide is also called 4-Nitroacetanilide para nitroacetanilide is a chemical compound that is a nitroacetanilide derivative prepared from acetanilide and nitrating mixture. Thus the order of basicity of the nitro substituted anilines follows m.
Aniline can be nitrated regioselectively at the para position if the nitrogen is first protected from protonation. Consider the following bases I o-nitrianiline II m-nitroaniline III p-nitroaniline The decreasing of basicity is. Fast Red P Base.
Acetylation of Aniline In the first step we need to put the removable acetyl protecting group on the nitrogen of aniline. The m-nitro aniline is unstable and so it can donate a pair of electron easily than the other two. P - nitroaniline.
This if treated with H2SO4HNO2CH3COOH will produce paranito nacetyl aniline which on hydrolysis will give para nito aniline. When liquid bromine Br 2 l is added to the aniline it gives 246-bromoaniline. Fast Red MP Base.
To make p-nitroaniline from aniline through a three- step synthesis Fist Step acylation of aniline second step Nitration of Acetanilide. It is mainly used as a precursor to o-phenylenediamine. On the other hand p-nitroaniline.
Since aniline contains the amino group -NH 2 then it will be activating. The general idea of a protecting group was introduced in Sec. Figure 141 Three Step Preparation of p-Nitroaniline aniline 1.
Due to the presence of the electron-withdrawing nitro group the delocalization of the lone pair of electron is improved.
Experiment 14 Preparation Of P Nitroaniline Myweb
Https Magritek Com Wp Content Uploads 2020 03 Lab Manual Synthesis Of P Nitroaniline Web Pdf

Nitration Of Aniline Chemistry Stack Exchange
Why Is It Impossible To Prepare P Nitroaniline Directly From Aniline Quora
How Can One Prepare P Nitroaniline From Aniline Quora
Komentar
Posting Komentar