Para Vs Ortho Hydrogen
Ortho nitrotoluene is more reactive than para nitrotoluene towards Electrophilic Substitution reactiontherefore being less stable the reason being that though both Ortho and para directing groups are activating groupsie they increase reactivity of the compound towards the above reaction para position is less activating than Ortho ie is more stableie less reactive the. Ii Boiling point of para hydrogen 2026K while that of.
Determination Of Ortho And Para Hydrogen Ratio By Using Raman Spectroscopy Jasco Global
09072020 Ortho and Para hydrogen Hydrogen molecule in which two hydrogen atoms have same nuclear spin parallel nucleus spin is called ortho hydrogen and hydrogen molecule in which two hydrogen atoms have opposite nuclear spin anti-parallel nucleus spin is.
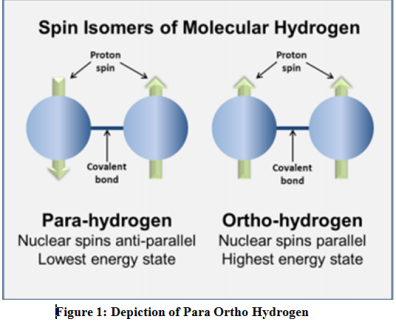
Para vs ortho hydrogen. Ortho-hydrogen molecules are those in which the spins of both the nuclei are in the same direction. Therefore the energy of Para-Hydrogen is lower than that of Ortho-Hydrogen. Ortho and para hydrogen are similar in chemical properties but differ in some of the physical properties.
15082020 At the temperature of liquefaction of air the ratio of ortho and para hydrogen is 1. Ortho-Para Hydrogen Let J be the total angular momentum of a hydrogen molecule including spin. This is trouble because the ortho-para transition releases a modest amount of energy and it doesnt take much energy input to boil off.
At liquid-hydrogen temperatures the stable state is almost all para. At room temperature hydrogen is about 34 ortho. Para-hydrogen is when the spins of both the nuclei are in the opposite directions.
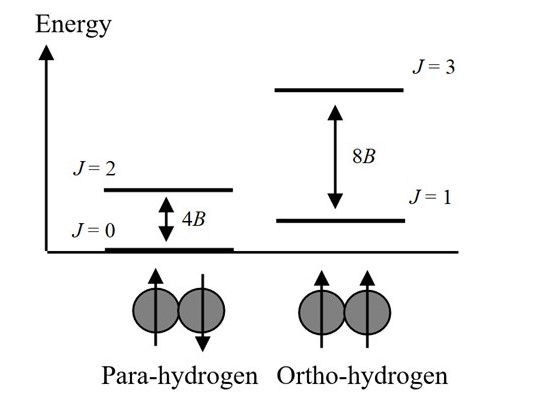
Even at very high temperatures the ratio of ortho to para hydrogen can never be more than 3. 10092019 The key difference between ortho and para hydrogen is that ortho hydrogen molecules have spins of two nuclei in the same direction whereas para hydrogen molecules have spins of two nuclei in opposite directions. A fundamental postulate in.
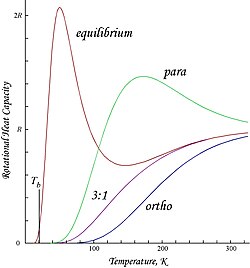
The distinction between the two isomers is the nuclear spin. Stability of ortho- and para-hydrogen 1 answer Closed 5 months ago. The amount of ortho- and para-hydrogen varies with temperature.
K IS about 254 calories per mole whereas the endo. 30082020 If both the nuclear spins are parallel the molecule is called ortho and if the spins are antiparallel it is referred to as para in disubstituted benzene para refers to the two groups at two opposite ends while in ortho they are adjacent or parallel to each other. We categorize them as such.
Hydrogen molecule is formed due to combination of two hydrogen atoms. The reason why the conversion between ortho-hydrogen oH2 and para-hydrogen pH2 is extremely slow is that they have differ-ent nuclear spin states. Thermic heat of vaporization of liquid hydrogen is 216 calories per mole 1 2 As a result of this slow change a thermally isolated tank of liquid hydrogen prepared without conversion to the para form will lose about 1 percent of its volume during the flrst day of storage.
Subscribe to our ChannelFacebook Page. The ortho-hydrogen o-H2 and the para-hydrogen p-H2. Eat of conversion of ortho-to parahydrogen at 20.
But the ortho-para conversion is slow so if you just liquefy hydrogen what you get is still 34 ortho and it slowly converts itself to para. Plus as we know that the everything in nature tries to attain lowest possible energy level and ortho form with opposite spins counteracts each others magnetic moments. Thus it has been possible to get pure para hydrogen by cooling ordinary hydrogen gas to a very low.
At the room temperature the ratio of ortho to para hydrogen is 3. The spin on the hydrogen nucleus has a magnitude of. The intrinsic energy of ortho is greater than para -hydrogen and also with rise in temperature of ortho form increase while para decreases and ultimately at room temperature its 75 ortho and 25 para -hydrogen.
The S 0 line near 360 cm -1 is formed by the transition between para levels and the S 1 line near 590 -1 is formed by the transition between ortho levels. The two protons present in the nucleus of both the hydrogen atoms may spin either in the same direction or in opposite directionThus depending on the direction of the two proto. 21092020 Hydrogen has two different spin isomers.
Molecular hydrogen in a hydrogen molecule H 2 can be found in two forms as ortho hydrogen and para hydrogen. Ordinary hydrogen gas is an equilibrium mixture of ortho and para hydrogen. The two protons of ortho-hydrogen spin in the same direction and the two protons of para-hydrogen spin in the opposite direction.
It is known that Para-Hydrogen has opposite spin and Ortho-Hydrogen has same spin. Nuclear spins interact so weakly with other properties of matter that these spin states are conserved in almost any process including chemical reactions. Ortho states have odd J 1 3 and para states have even J 0 2.
I Melting point of para hydrogen is 1383K while that of ordinary hydrogen is 1395 K.
Vortex Tube Innovation For Sustainable Energy Washington State University

Why Do Spin Isomers Of Hydrogen Ortho And Para Hydrogen Change Their Nuclear Spin With Temperature Variance Chemistry Stack Exchange

Kinetics And Heat Exchanger Design For Catalytic Ortho Para Hydrogen Conversion During Liquefaction Donaubauer 2019 Chemical Engineering Amp Technology Wiley Online Library
Spin Isomers Of Hydrogen Wikiwand

Why Equilibrium Hydrogen Doesn T Exist Hydrogen Properties For Energy Research Hyper Laboratory Washington State University
Komentar
Posting Komentar