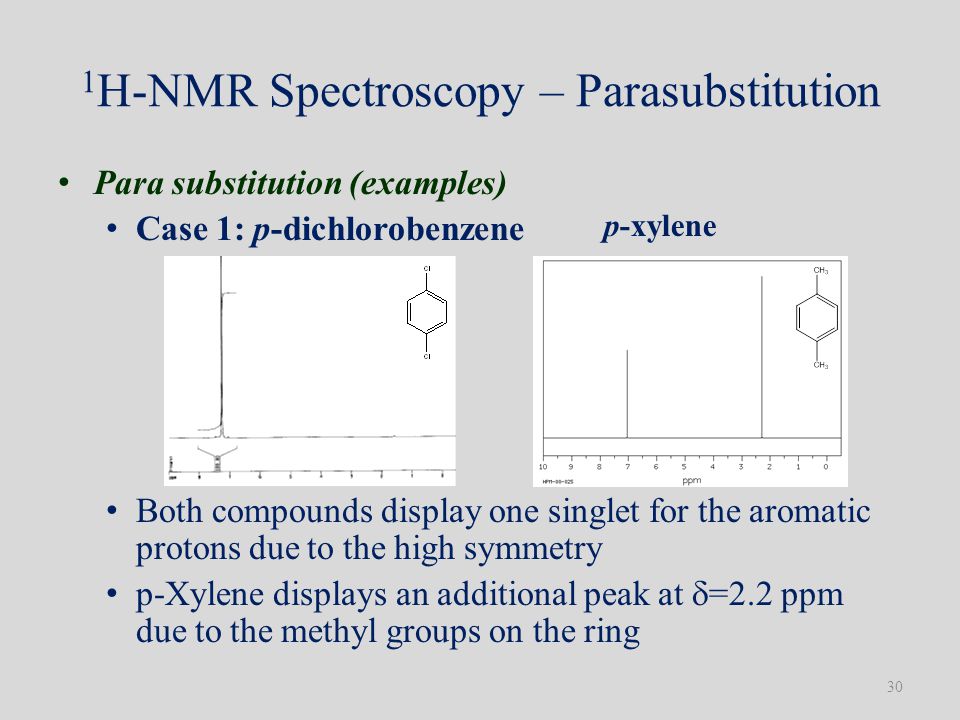
Para Xylene Nmr Signal
Ortho-xylene Meta-xylene And Para- Xylene. 11082020 The 1 H-NMR spectra that we have seen so far of methyl acetate and para-xylene are somewhat unusual in the sense that in both of these molecules each set of protons generates a single NMR signal.
Introduction To Nmr Spectroscopy Ppt Download
The 1 H-NMR spectra that we have seen so far of methyl acetate and para-xylene are somewhat unusual in the sense that in both of these molecules each set of protons generates a single NMR signal.
Para xylene nmr signal. Take a look next at the spectrum of para-xylene IUPAC name 14-dimethylbenzene. 40 Ca 13C NMR Spectroscopy of Aromatic Compounds As with other 13C NMR spectra aromatic compounds display single lines for each unique carbon environment in a benzene ring. Ignore The CDC3 Signal Spectrum A сHз.
Molecular Weight 13619. The only peak that comes before saturated C-H protons is the signal of the protons of tetramethylsilane CH3 4 Si also called TMS. The integration function can also be used to determine the relative amounts of two or more compounds in.
You can use 13C NMR for distinguishing o-m- and p-sub. Aromatic carbons appear between 120-170 ppm. Rather than being a complication however this splitting.
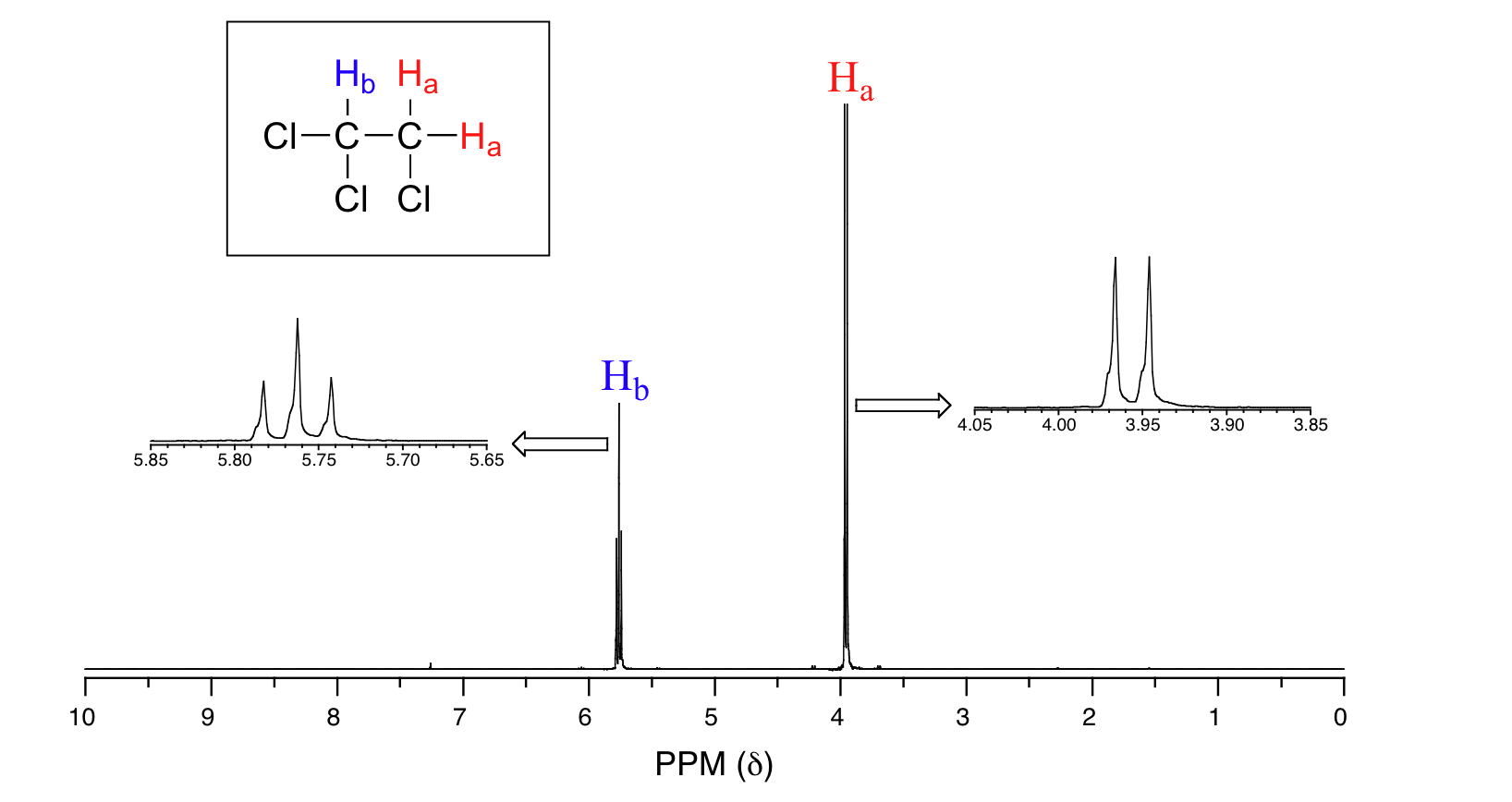
- signal is. Tells us which set of protons corresponds to which NMR signal. Unlike 1 H-NMR signals the area under a 13 C-NMR signal cannot be used to determine the number of carbons to which it corresponds.
Nuclear Magnetic Resonance NMR Nuclei behave like a bar magnet. Compound p-Xylenewith free spectra. In fact the 1 H-NMR spectra of most organic molecules contain proton signals that are split into two or more sub-peaks.
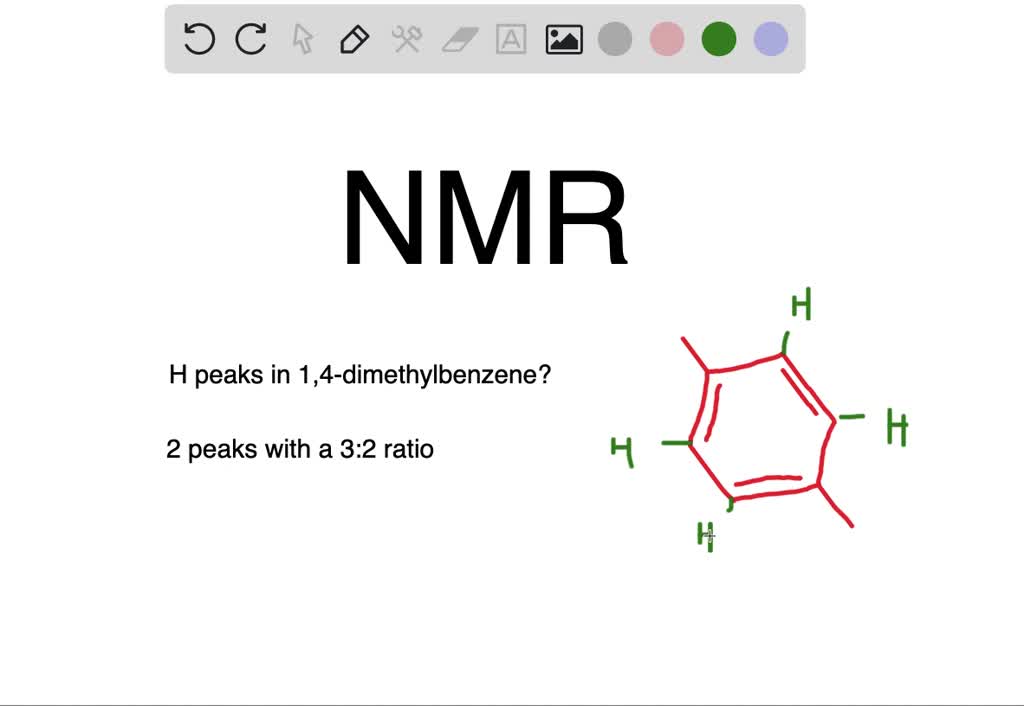
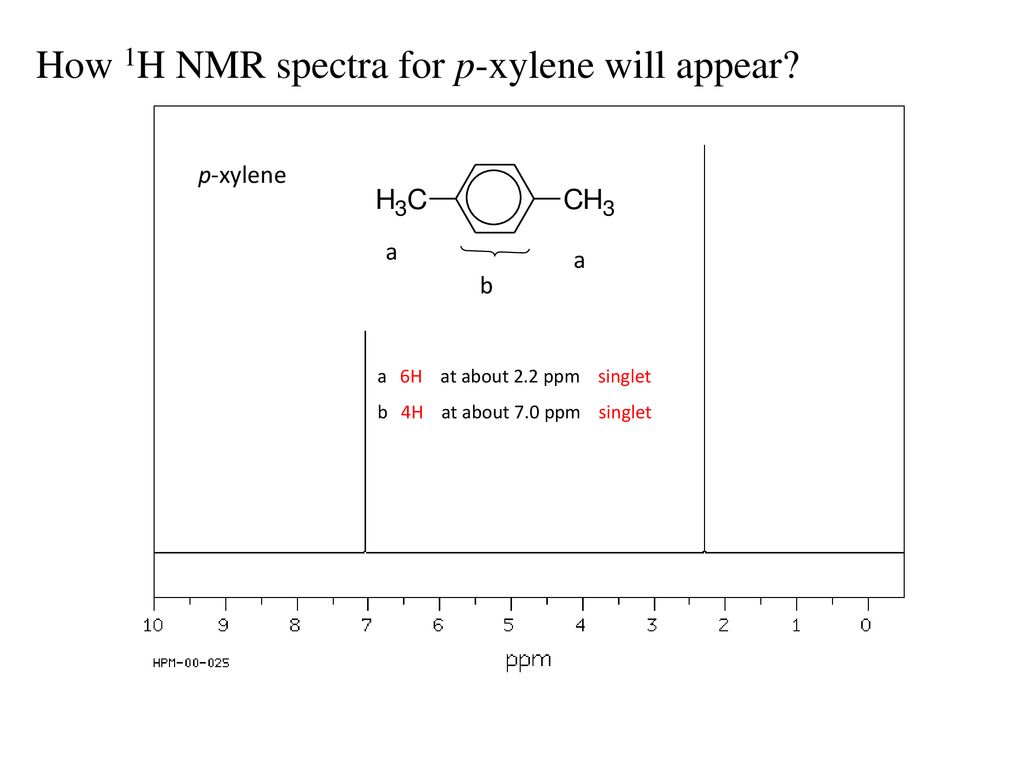
4 6 704 229 parts per million ppm 14-Dimethylbenzene para-Xylene The 1H-NMR spectrum C 8 H 10. The chemical shifts where the signals appear Section 135 Most complex facet. The number of signal sets Section 136 The number of signal sets tells how many types of symmetry-unique hydrogen are present Symmetry-duplicate hydrogens give the same signal sets 2.
Analyzing para-Xylene H b H b H b H b H b H b H H b H a H a H a H a H a H a H a H a H a H a H a a C 8 H 10 para-Xylene. This is because the signals for some types of carbons are inherently weaker than for other types peaks corresponding to carbonyl carbons for example are much smaller than those for methyl or. This molecule has two sets of protons.
The four facets of 1H NMR spectroscopy. 13C NMR PROBLEM 9 Match Each Spectrum To The Isomers Of Xylene. In fact the 1 H-NMR spectra of most organic molecules contain proton signals that are split into two or more sub-peaks.
60 NMR 19 FTIR 2 Raman 2 Near IR and 23 MS 1 H Nuclear Magnetic Resonance NMR Chemical Shifts View the Full Spectrum for FREE. BeilsteinREAXYS Number 2206190. P-Xylene View entire compound with free spectra.
Whether NMR data are provided as in Figure of NMR Spectrum of 14-dimethylbenzene with an integral step over each signal or simply with numbers that represent each signals relative area the process of interpreting the data is the same because the area of each signal is proportional to the number of hydrogen atoms producing that signal. 11082020 The basics of 13 C-NMR spectroscopy. Linear Formula C 6 H 4 CH 2 NH 2 2.
The 13C NMR spectra of bromobenzene and p-bromoethylbenzene are shown below for comparisonThere are four different carbon. When we instruct the instrument to integrate the areas under the two signals we find that the area under the peak at 26 ppm is 15 times greater than the area under the peak at 74 ppm. This is a standard reference point with the signal set exactly at 0 ppm and you can ignore it when analyzing an NMR spectrum.
Groups considering benzene as a aromatic substrate. 26022021 For example of IUPAC 14-dimethyl benzene para-xylene spectrum which can be seen that the molecule has two protons namely six methyls Ha protons and four aromatic protons Hb. The six methyl H a protons and the four aromatic H b.
The instrument integrated the area under the two signals and it was found that the area at the peak at 26 ppm was 15 times greater than at 74 ppm. 14-Bisaminomethyl benzene αα-Diamino-p-xylene CAS Number 539-48-0. CH3 CH3 CH3 CH3 0-xylene M-xylene CH3 P-xylene 268 180 Spectrum B 20 100 Spectrum C.
4 6 704 229 parts per million ppm. For ortho groups of an aromatic molecule will give 3 signals for Carbons. Rather than being a complication however this.
PubChem Substance ID. 02022021 In industry scientists usually employ distillation fractional crystallization and adsorption in high temperature and high pressure environments to separate the isomers which are individually referred to as para-xylene p-xylene or PX meta-xylene m-xylene or MX or ortho-xylene o-xylene or OX. There are a lot of compounds especially organometallics that give signal at negative ppm but you will probably.
60 NMR 19 FTIR 2 Raman 2 Near IR and 23 MS. Take a look next at the spectrum of para-xylene IUPAC name 14-dimethylbenzene. The six methyl H a protons and the four aromatic H b protons.
This molecule has two sets of protons.

Nmr Spectroscopy Lecture 5 Substitution Patterns On Aromatic Rings Part 5 Of 5 Youtube
5 14 Spin Spin Splitting In 1h Nmr Spectra Chemistry Libretexts
Https Www Chem Wisc Edu Deptfiles Orglab Handouts Chem 20344 201h Nmr 20lecture 201 20spring 202014 Notetaking Pdf
Solved How Many Peaks Would You Expect In The H
1h Nmr Spectra Interpretation Ppt Download
Komentar
Posting Komentar